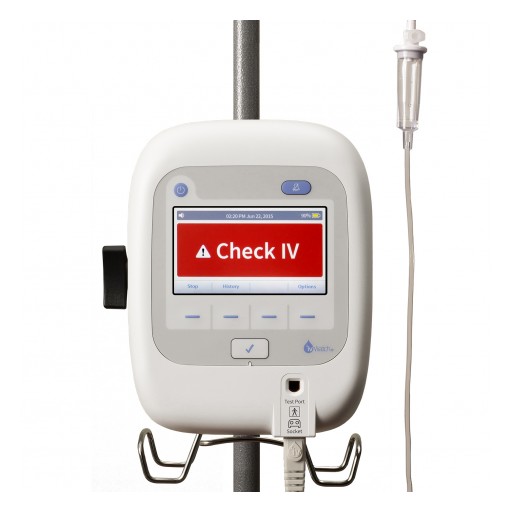
Latest Press Releases
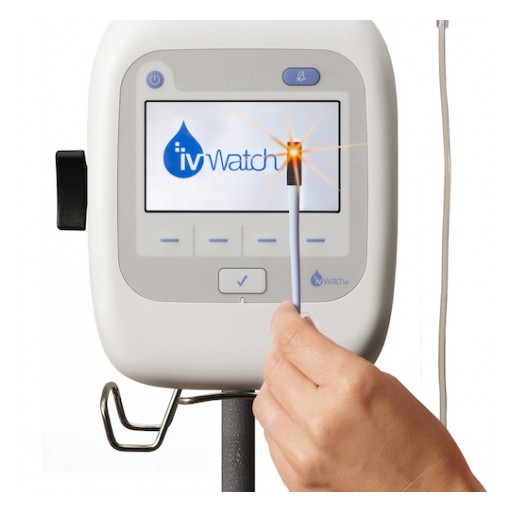
Terumo Corp., exclusive distributor for ivWatch in Japan, licenses ivWatch infiltration detection technology for use in products globally


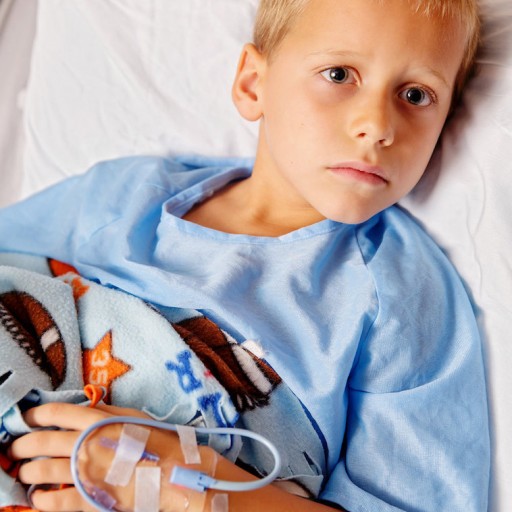
New indication allows for wide scale implementation to help protect children across the United States
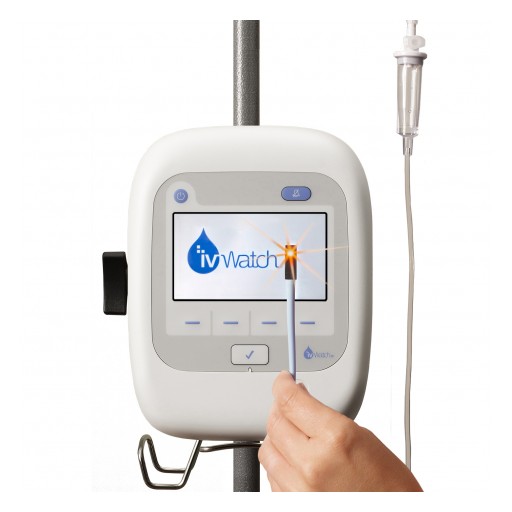
The ivWatch Model 400 will improve patient safety through early detection of IV infiltrations and extravasations and provide a new option for IV therapy.